Electric Double Layer & Charge Distribution at Particle Surfaces
DanielRGrisham, CC BY-SA 4.0, via Wikimedia Commons
The atomic behaviors of particles and substances in specific conditions is a field of study that provides endless interest for casual and professional students of physics. One such phenomenon is the formation of an electric double layer.
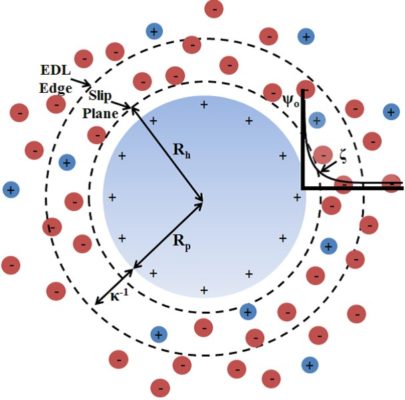
The electric double layer refers to a dual layer of positively charged ions that adhere to and surround an object submerged or in direct contact with liquid. The initial layer of positive ions forms a tight layer around the physical object in question, while the second layer exists in the surrounding liquid just beyond the initial ion layer, attracted by the residual pull of the negatively charged physical object.
The study of both layers helps us understand how particles behave in liquid and how particles attract and interact between substances.
Keep reading to learn more about this minute yet fascinating occurrence, theories as to how and why the electric double layer forms, and its impact on current and future technology.
How Does the Electric Double Layer Work?
The world around us is charged. Quite literally, the physical world in all of its forms is composed of positive and negative electric charges, and how these charges interact with one another affects the natural world and is itself affected by natural laws.
Negative and positive charges will attract, whereas two positive or two negative charges will repel away from one another, similar to the workings of a magnet. When an object’s surface experiences ionic burden in a particular way it will tend to attract surrounding ions of opposite charge.
In liquid contexts, this initial layer of ions will tend to crowd the surface of the object in a very tight layer, behaving almost as a second skin. An object submerged in liquid innately acquires a negative charge. This negatively charged solid object will immediately draw to itself positively charged ions within its liquid environment, which will compress in a tight initial layer on the object’s surface.
Taojiang12345, CC BY-SA 4.0 <0>, via Wikimedia Commons
This ring of positively charged ions, however, is not tight enough to completely block out or negate the negative charge of the object beneath, and a secondary, looser layer of positive ions gathers directly above.
This natural balance of negative and positive charge surrounding a submerged object helps substances and properties within liquid remain distinct from their surroundings, and can even allow for new substances to form within a changing liquid context.
Various models or theories have been put forward to attempt to understand and predict the behavior of the ions composing the electric double layer.
Different Models Exist to Explain the Electric Double Layer
While this phenomenon of the electric double layer has been widely observed, seeking to understand why ions in liquid behave in this manner has given rise to several theories.
One such theory is the Gouy-Chapman model which takes into account the thermal motion and the electrostatics of the ions themselves. This model proposes that the double layer is a result of the natural motions of molecules within the electrolyte in question, and is not a rigidly set distance.
However, this model tends to underestimate the densities of electric double layers and is not perfect.
The DLVO theory rests on the assumption that forces of repulsion between ions determine the creation and behavior of the electric double layer, and this model can be adjusted to application in different electrolytes. It takes into account the size and shape of colliding particles as well as the liquid in which the object is immersed.
This theory struggles to account for the behavior of colloidal crystals, however, and can not give an account for the formation of colloidal crystals within saline contexts.
The Electric Double Layer Has Deeper Implications for Various Fields
Whatever the causes of the electric double layer may be, its physical reality lends much to the study of other fields of science and technological development.
Consider colloid science (the study of liquid mixtures containing solid particles which remain suspended). Thanks to the existence of the electric double layer, materials within a liquid can exist in a suspended or a “floating” state, if you will, hanging within and throughout the material and giving it a cloudy appearance.
Understanding the electric double layer can help us better observe and report on electrokinetics, or how particles move in an electrified or charged environment. This field of study may have future applications as we seek to develop alternative sources of energy, more efficient and cleaner batteries, and less wasteful building materials, too.
The behavior and existence of the electric double layer are also interesting in light of the impacts that electricity has on matter on an incredibly small scale, i.e. within electrochemistry. As our technology becomes smaller and smaller, understanding the behavior and dynamics of the electric double layer will have implications for the implementation of nanotechnology, and our success in manipulating matter and electricity on a microscopic scale and within the human body, as well.
Understanding the electric double layer also plays a role in emerging biochemical detection technologies, useful in health and medical contexts (such as detecting an illness through sweat analysis), as well as applicable in national security operations.
Silver’s Role in Charge Distribution at Particle Surfaces
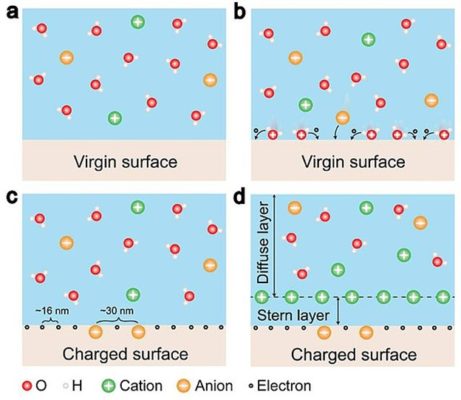
Silver, a transition metal from the periodic table, boasts some unique properties, one of which is the formation of an electric double layer (EDL) at its surface. This EDL is a thin layer of charges that plays a crucial role in determining the overall charge distribution of the particle.
Scientists use electrochemistry and electron microscopy to study the EDL around silver particles. These techniques help scientists understand the properties of the EDL, like the thickness of the layer and the types of ions present.
Knowing the properties of the EDL around silver particles is important for a number of industrial and scientific applications.. For example, silver particles are used in electronic devices like conductive inks and pastes. If we understand the EDL it can help make these devices work better. Also, silver particles are used in biomedical applications, like drug delivery. Understanding the EDL can help make these applications better too.
Conclusion
The electric double layer refers to the double layers of positively charged ions that surround the innately negatively charged object that has been submerged in a fluid. The initial layer of ions forms a tight ring or skin around the object, and a secondary layer of positively charged ions hovers directly above, drawn in by the faint negative charge emitted by the underlying object.
The Gouy-Chapman model seeks to understand the behavior of these ions in terms of thermal motion and the natural kinetics of the charged molecules within the liquid environment. The DLVO theory, in contrast, seeks to explain the existence of the double layer in terms of the innate push and pull between negatively and positively charged ions.
Gaining a better understanding of the electric double layer can be an immense help in the fields of clean energy, nanotechnology, the study of suspended or colloid materials, and medical biotechnology. Doubtless this simple yet essential principle of surface science will have applications yet to be discovered in the future as health science, security technology, and electronic advancements increasingly intersect.
Featured Image Credit: By Taojiang12345 – Own work, CC BY-SA 4.0,
In Post Image 1 Credit: Public Domain, via Wikipedia Sources Used :
https://www.youtube.com/watch?v=HDQ8ct4md-8
https://www.sciencedirect.com/topics/engineering/gouy-chapman-theory
https://link.springer.com/referenceworkentry/10.1007/978-1-4419-6996-5_7
https://www.youtube.com/watch?v=15PkDYSoS6s