Understanding the Surface Charge & Ionization of Surface Groups
1.Introduction
When two conductive substances in different states of matter interact, they generate a surface charge — the electrical potential difference between them at the interface where they meet. This charge has significant implications for the interactions between the two materials and is key to many fields of study.
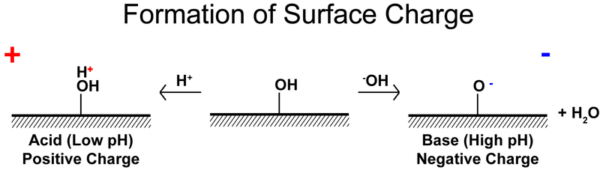
Arsteven, CC BY-SA 3.0, via Wikimedia Commons
While a surface charge can be manipulated through pathways such as the adsorption of ions or the preferential dissolution of a solid, the most straightforward means of controlling it is the ionization of surface groups.
Surface charge plays a particularly important role in areas of chemistry like colloids, coatings, proteins, and pharmaceuticals. When not accounted for, solids that generate undesired surface charges can behave in unexpected ways—they may coagulate, adsorb unwanted contaminant ions, or be unable to interact properly with their target cells or proteins. But when leveraged as a tool, surface charge can not only prevent these undesirable side effects but allow a significant degree of control over the interface between the two states of matter.
In this article, several important features of surface charge and surface group ionization will be covered, including how surface group ionization works, how surface charge can be measured, and how these techniques can be applied to further all kinds of cutting-edge technology.
2. Basics of Surface Charge
Surface charge is mainly useful in controlling the behavior of an interface of two materials in different states of matter. While these could be any two states, it is most commonly useful in examining solid-liquid interactions. This could be a pharmaceutical in the bloodstream, many small solid particles suspended in liquid as a colloid, or simply water on a car. If these two materials are both conductive, an electrical potential difference is generated. This potential difference will affect the behavior of both the solid and liquid and is distinct from the overall charge, or bulk charge, of the entire solid and entire liquid.
Take, for example, a negatively charged solid. In solution, positively charged ions will be attracted to and often adsorbed to the negative surface.
This layer is known as the Stern layer and is considered immobile for all intents and purposes—a part of the solid. This positive outer layer now interacts with the remaining ions in the solution, forming a diffuse layer or shear plane of loosely associated ions.
These ions vary in charge but are usually predominantly the opposite charge of the Stern layer—negative, in this example. At the boundary between these two layers, known as the electric double layer, the electrokinetic potential of the interface can be measured. This potential is known as the interface’s zeta potential, and it dictates the effective charge of the solid surface in solution, and how it will behave.
Additionally, the zeta potential is not always the same charge sign as the original solid surface. Polyvalent or surface-active counterions can form a Stern layer that attracts ions of the opposite charge of the solid surface to the shear plane, and nonionic surfactants can be adsorbed to the
solid surface to prevent a zeta potential from being generated at all. These techniques are often used to manipulate or eliminate undesirable zeta potential.
Surface charge can affect the behavior of a solid or particle in a solution in many ways. A colloid with sufficient zeta potential will have enough charge to repel other colloid particles and remain suspended; if the zeta potential is too low, the particles will not repel each other strongly enough and will coagulate. A properly formulated coating can prevent metals exposed to liquids from interacting with ions that might oxidize them or otherwise react with them. Surface charge even has complex implications for environmental fields like soil chemistry, where the charge of the soil’s surface affects key factors like nutrient retention.
There are several ways a surface charge can be deliberately controlled to ensure desired solid-liquid interactions. One of these is physical—an electrical charge applied to the solid—but this is often impractical or impossible. The others are chemical and come in three particularly common approaches.
The first is the adsorption of charged species. This approach adds charged surfactants to a solution to take advantage of the adsorption of ions to a solid surface submerged in a liquid. These surfactants will preferentially adsorb to the solid, giving it a negatively or positively charged surface, depending on the charge of the surfactant ion.
The second is the differential loss of ions. When solids are placed in solution, they sometimes dissolve unevenly, losing more of one of their ions to the surrounding solution than the other. This creates a charge in the solid, which is now composed of more ions of one charge than the other, and will interact with the surrounding solution accordingly. Both the composition of the solution and the solid can be modified to achieve this differential dissolution.
the third and most straightforward means of controlling surface charge is by formulating it from or coating it with materials containing surface groups that can be selectively ionized to create the desired charge. Through this deliberate ionization of surface groups, the surface charge of two interacting materials can be controlled. This method has the distinct advantage of being built into the material in question. Since it requires no additives to the liquid and can range from a direct property of the solid to a specialized coating, the ionization of surface groups offers the most flexibility and the greatest degree of control.
1.Ionization of Surface Groups
Amino acids are common compounds with ionizable groups. At different temperatures and pH, these ionizable groups dissociate, changing the overall charge and functionality of the molecule. It is this flexibility that makes them such a useful building block for life.
Ionizable groups at the surface of a material behave the same way. The behavior and charge of these surface groups will go on to determine the surface charge of the solid, and therefore its behavior. They can even be tailored to respond to certain conditions.
pH is the most important factor in determining the function of ionizable surface groups. At low pH, hydronium ions will be available to protonate surface groups and accept hydroxide ions, creating a positively charged solid that will tend to form a negative Stern layer. The opposite is true at high pH.
The exact degrees of these charges and the resulting zeta potential depend on the surface groups in question, the other ions in the solution, and the pH of the overall solution, giving a large degree of flexibility in creating specific surface charges. Temperature also plays a role in regulating the ionization of these groups, and therefore the surface charge.
Through the deliberate creation of solids, particles, and coatings with ionizable surface groups that give them a positive or negative charge in certain conditions, many solid-liquid interactions can be controlled and prepared for. A particle designed for use in a particular solution can be rendered negatively charged so it does not bind to a necessary negative ion in that solution. A coating can be applied to a surface that repels ions of a certain charge, preserving its integrity. A highly negative surface can discourage bacterial growth. But to control these properties, one must first be capable of measuring them.
3.Surface Charge Measurement Techniques
To properly control the interaction of a solid with a solution, its functional charge in that solution must be known. Since zeta potential has a linear relationship with the actual surface potential, both can be measured to determine the surface charge. There are currently three main established methods of measuring surface charge, one measuring the actual surface potential, and two measuring the zeta potential.
Kelvin Force Probe Microscopy (KFM)
This method measures the actual surface potential of a solid sample, usually submerged in liquid. In KFM, a probe is contacted with the surface to measure the potential difference between the
sample and probe tip. This method is used for a wide number of materials, both inorganic and organic, and in both polar and nonpolar solutions. Since the sample must be large and secure enough for the probe to contact, it is primarily used for solid surfaces. While broadly useful, this method has limitations when it comes to irregular samples, which decrease accuracy, and small samples like colloids or proteins, which it cannot measure.
Streaming Potential
This method embeds particles of the sample solid in a substrate, then passes various streams of ionic liquids over the substrate, measures the resulting potentials, and uses these to calculate the various zeta potentials. It is commonly used in characterizing the behavior of a wide variety of materials like wood, fibers, inorganic surfaces, and colloids. Since it requires the solid to be immobilized in a substrate for testing, this method is unable to characterize the interactions of a specific solid and liquid in the field, unlike KFM and optical and acoustic detection.
Optical and Acoustic Detection
When a current is applied to particles in solution, these particles experience electrophoretic motion and move towards the electrode corresponding to their opposite charge. The speed at which they move is characteristic and can be used to calculate their zeta potential. The speed of the particles is most easily detected optically, but in cases where the sample liquid is cloudy and light cannot penetrate it, electroacoustic detection is used to generate movement of the particles instead, and the resulting potential is measured. These methods require uniformly distributed and appropriately diluted samples, but offer a large sample size and minimal sample preparation. They are used to study solids suspended in solution, such as colloids, nanoparticles, proteins, and pharmaceuticals.
While these are the most established means of measurement now, others are in development. Devices like chemical field effect transistors (chemFETs) are tiny structures with small gates. When a liquid passes through these gates, a characteristic potential is generated and measured directly by the transistor. Also in development are specialized materials like nanopores, which are extremely sensitive to particular surface charges generated in the presence of certain ions in solution, making them effective sensors.
5.Applications of Surface Charge and Ionization of Surface Groups
To properly control the interaction of a solid with a solution, its functional charge in that solution must be known. Since zeta potential has a linear relationship with the actual surface potential, both can be measured to determine the surface charge. There are currently three main established methods of measuring surface charge, one measuring the actual surface potential, and two measuring the zeta potential.
Kelvin Force Probe Microscopy (KFM)
This method measures the actual surface potential of a solid sample, usually submerged in liquid. In KFM, a probe is contacted with the surface to measure the potential difference between the sample and probe tip. This method is used for a wide number of materials, both inorganic and organic, and in both polar and nonpolar solutions. Since the sample must be large and secure enough for the probe to contact, it is primarily used for solid surfaces. While broadly useful, this method has limitations when it comes to irregular samples, which decrease accuracy, and small samples like colloids or proteins, which it cannot measure.
Streaming Potential
This method embeds particles of the sample solid in a substrate, then passes various streams of ionic liquids over the substrate, measures the resulting potentials, and uses these to calculate the various zeta potentials. It is commonly used in characterizing the behavior of a wide variety of materials like wood, fibers, inorganic surfaces, and colloids. Since it requires the solid to be immobilized in a substrate for testing, this method is unable to characterize the interactions of a specific solid and liquid in the field, unlike KFM and optical and acoustic detection.
Optical and Acoustic Detection
When a current is applied to particles in solution, these particles experience electrophoretic motion and move towards the electrode corresponding to their opposite charge. The speed at which they move is characteristic and can be used to calculate their zeta potential. The speed of the particles is most easily detected optically, but in cases where the sample liquid is cloudy and light cannot penetrate it, electroacoustic detection is used to generate movement of the particles instead, and the resulting potential is measured. These methods require uniformly distributed and appropriately diluted samples, but offer a large sample size and minimal sample preparation. They are used to study solids suspended in solution, such as colloids, nanoparticles, proteins, and pharmaceuticals.
While these are the most established means of measurement now, others are in development. Devices like chemical field effect transistors (chemFETs) are tiny structures with small gates. When a liquid passes through these gates, a characteristic potential is generated and measured directly by the transistor. Also in development are specialized materials like nanopores, which are extremely sensitive to particular surface charges generated in the presence of certain ions in solution, making them effective sensors.
Applications of Surface Charge and Ionization of Surface Groups
Ccertain surface groups. This ionization process can be used to investigate the surface of various materials, which is important for many industrial and scientific applications.
In particular, silver is widely used in surface analysis techniques such as X-ray photoelectron spectroscopy (XPS) and Auger electron spectroscopy (AES) to measure the surface chemical composition, oxidation state, and electronic properties of materials. These techniques rely on the ability of silver to ionize surface groups, which allows scientists to probe the electronic structure of the surface and understand how it interacts with other materials.
Additionally, silver ions possess antimicrobial properties, making them a useful material in the medical industry. Silver ions can be used to kill a wide range of microorganisms, including bacteria, viruses, and fungi, making the metal a powerful tool in the fight against infection.
Conclusion
Surface charge and the ionization of surface groups are concepts key to the understanding of solid surfaces and their interactions with solutions. As measurement techniques and the ability to manipulate surface charge advance, so does the ability of chemistry to fine-tune the complex interfacial reactions between states of matter. This area of materials chemistry will be of critical importance to coming developments in biochemistry, coatings, catalysts, colloids, nanotechnology, environmental science, and more.
References